Initial efforts in the Clemmer group were directed at understanding the nature of protein conformation in the gas phase using a combination of physical (collision cross section measurements) and chemical probes (H/D exchange and proton transfer reactions). What follows is a brief discussion of some of the early work in these fields that has been performed in the Group. Much of this work has been — and continues to be — updated on a regular basis, however, this initial work has been carried over from the original versions of the website for historical and posterity reasons.
Furthermore, a movie containing animated data showing the unfolding of cytochrome c ions in an ion trap can be found here.
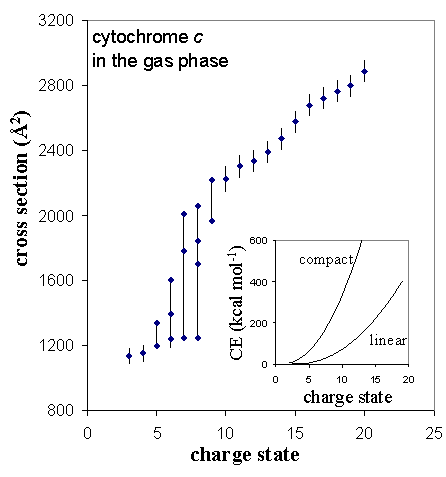
Protein ions formed by electrospray ionization usually are observed as a distribution of charge states. As charge state increases for a given protein, the coulombic repulsion energy must also increase. For high charge states, an insufficient number of intramolecular stabilizing interactions are possible to maintain a compact conformation. The result is coulomb-induced unfolding.
As an example of this, the collision cross sections observed for cytochrome c are plotted below as a function of charge state. For low charge states (3+ and 4+), the cross section is close to that calculated for coordinates of the protein crystal structure. For charge states 5+ to 9+, multiple conformations are observed, indicating a range of progressively more unfolded conformers. Higher charge states (≥ 10+) have cross sections that correspond to relatively open conformations that have little or no tertiary structure.
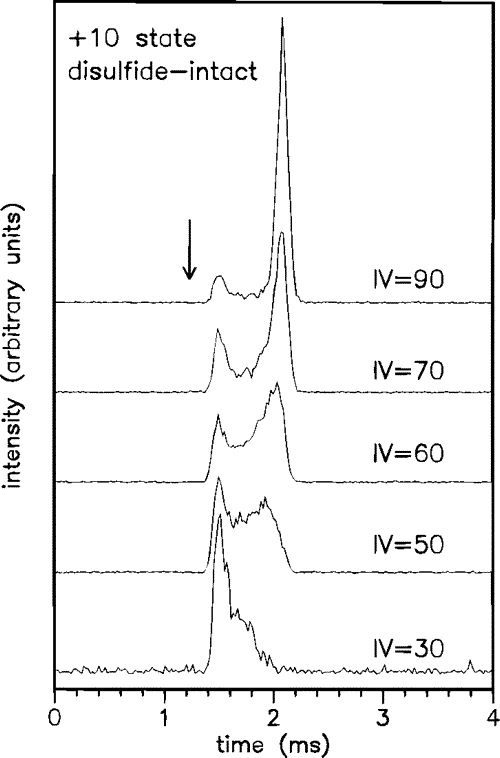
This figure shows the effect of varying the energy (IV, Injection Voltage (V)) with which lysozyme 10+ charge state ions are injected into the drift tube. At higher injection energies, the protein ions unfold to adopt more open conformations. The arrow indicates the drift time calculated for transport of the crystal structure of lysozyme through the drift tube.
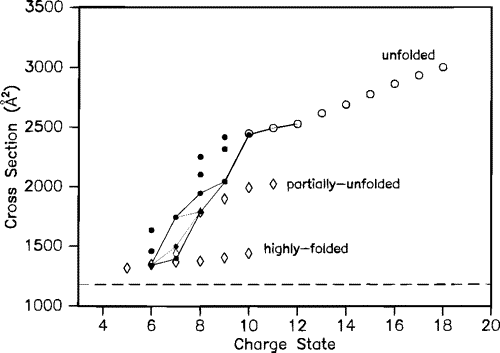
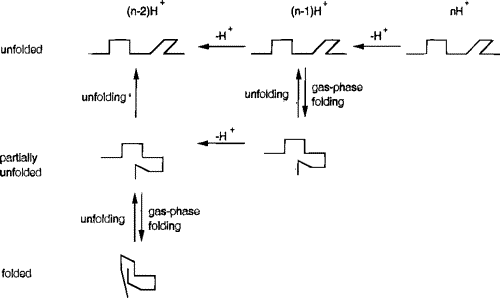
Studies of disulfide-reduced lysozyme showed that removal of protons by proton-transfer reagents induced folding. The collision cross sections shown below are connected with lines to indicate possible folding pathways inferred from the proton transfer studies.
Early ESI studies of proteins by Chait and others demonstrated that the charge state distributions were dramatically influenced by solvent conditions. We used the protein ubiquitin as a model system to follow the conformational changes upon ESI from mostly aqueous (90%) and mostly denaturing (90% acetonitrile) solutions.